
TBS INDIA aspires to the highest standards of excellence and professionalism in the provision of high-quality care that is safe, effective and focused on customer experience in the people it employs, and in the maintenance support, education, training and development they receive in the leadership and management of its organizations and through its commitment to innovation and to the promotion, conduct and use of research to improve the current and future service to the full satisfactions of the customer.

Quality Management Systems are processes, sequences and interactions plus any resources which can be used to efficiently manage a Biomedical department. By monitoring, measuring and analyzing, controls can be put into operation throughout the system to ensure a high level of performance and service provision.
TBS INDIA have implemented, and currently run, successful Quality Management Systems to ensure that our customers receive high standards of medical device management.
Quality Management systems attract key benefits to Biomedical departments in terms of management control, reporting, measurable objectives and consistent quality of service delivery.

Quality Management Systems are based on continual improvements. As we are matured enough in practicing the Quality management system, we want much more improvement in our core field (Biomedical). So we achieved the certification of ISO 13485:2016 – Quality Management System for Medical devices.
Some of the services we can offer:

By an appraisal of your systems and devices we can standardize your procedures across the wards and device holdings:

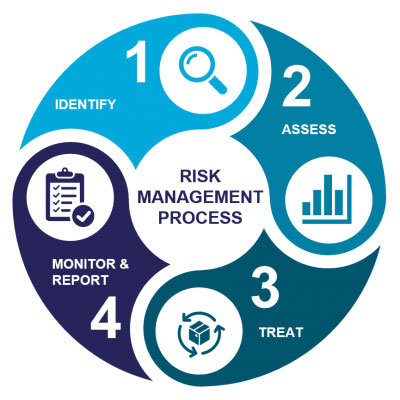

We can offer: